Diabetes mellitus, also called diabetes, is a group of metabolic diseases with high blood sugar levels over a prolonged period. Frequent urination, extreme thirst, and increased appetite are signs of elevated blood sugar. Diabetes, if left untreated, leads to several complications. Acute complications can include diabetic ketoacidosis and nonketotic hyperosmolar coma; chronic complications include cardiovascular disease, chronic renal failure, retinopathy, and damage to the feet.
Antidiabetic medicines control type 2 diabetes mellitus (T2DM).
This is a complete article on oral antidiabetic drugs and their uses. This article also shows you their mechanism of action, side effects, and treatment methods. In addition, we’ll cover the diverse types of medicines available and how each works to help your body manage your blood sugar levels.
- Antidiabetic Drugs Classification:
- Antidiabetic Drugs that increase Insulin Secretion – Insulin Secretagogues:
- D-Phenylalanine Derivatives:
- Drugs that lower blood glucose by their cellular action:
- Drugs that decrease insulin resistance – THIAZOLIDINEDIONES
- Drugs that inhibit Glucose Absorption:
- Drugs affecting Incretin effect :
- Drugs that inhibit glucose reabsorption from the Kidneys:
- Miscellaneous Antidiabetics:
- Ending Note:
Antidiabetic Drugs Classification:
There are many mechanisms by which antidiabetic drugs accts and reduces high blood glucose levels. Some medicines
- Increase insulin sensitivity
- Decrease insulin production from the liver
- Act on the pancreatic cells to produce more insulin,
- Lower the absorption of insulin from the intestine,
- Inhibit the reabsorption of glucose from the kidney.
Antidiabetic Drugs that increase Insulin Secretion – Insulin Secretagogues:
These are drugs that bind with sulfonylurea receptors on the pancreas. The exact mechanism of action causes an increase in insulin release from pancreatic β cells known as insulin secretagogues. All insulin secretagogues have a similar mechanism of action, but they are different in chemical structure. Their chemical structure is used to categorize them. These include;
- Sulfonylureas
- Meglitinides
- D-Phenylalanine Derivatives
Mechanism of Action:
Insulin secretagogues in this category have a similar mechanism of action. They bind with Sulfonylurea receptors in the pancreatic β cells and stop efflux (outward movement from cell) of potassium, causing depolarization of cells. Due to this depolarization, voltage-gated calcium channels open, causing an influx (inward movement to cell) of calcium and insulin release from the pancreatic β cells.
Sulfonylureas:
All sulfonylureas have an S-aryl sulfonylurea group in their chemical structure, due to which they are named Sulfonylureas.
They are the second most commonly prescribed drugs and are the cheapest in treating Diabetes Mellitus type 2.
These decrease high blood glucose levels by increasing insulin production and secreting more insulin from pancreatic β cells.
Their classification is based upon their potency to bind to their receptor. First-generation are less potent than the second generation.
All Sulfonylureas are metabolized in the liver into inactive compounds, and acetohexamide is metabolized into a more active compound than the parent drug.
The urine excretes the metabolites of the first generation, and biles eliminate second-generation sulfonylureas.
These show reduction in microvascular complications associated with diabetes mellitus type 2.
Side Effects of Sulfonylureas:
- Hypoglycaemia is a common and significant side effect of this class. Hypoglycaemia is more common in those who omit meals, exercise hard, or lose a substantial amount of weight.
- Weight gain is also frequent, particularly during the first year of treatment. Improved glucose regulation and higher food intake with hypoglycaemia are two reasons for weight gain.
- First Generation Sulphonamides are excreted in the urine, so they are rarely used due to their prolonged biological and hypoglycaemic effects in patients with decreased renal function.
- These are contraindicated in patients having a sulfa allergy.
Agents include:
Sulfonylureas are divided into two classes
- First Generation Sulfonylureas
- Second Generation sulfonylureas
Following are the first generation and second-generation antidiabetic drugs included in sulfonylureas.

Meglitinide Analogues:
Antidiabetic drugs included in this category have a similar mechanism of action as those of sulfonylureas, but they do not have an s-aryl sulfonylurea group in their structure. Agents include in Meglitinides are;
- Repaglinide
- Mitiglinide (not used in the United States) approved in Japan.
These are absorbed from the intestine and are entirely metabolized into inactive compounds by the liver, can be safely used for patients with kidney impairment and in the elderly.
Meglitinide Analogues show a short insulin surge immediately after taking; due to this, they are given before food. Hypoglycaemia may occur in those patients who do not have adequate carbohydrates in their food or who took medicine but skipped their meal.

D-Phenylalanine Derivatives:
Nateglinide is a D-Phenylalanine Derivative
and has a similar mechanism of action as other sulfonylureas and meglitinide
analogues. It is also absorbed by the intestine and entirely inactivated by the
liver. It is also safe for use in patients with kidney disease and the elderly.
It also causes hypoglycaemia and weight
gain.

Drugs that lower blood glucose by their cellular action:
Metformin a BIGUANIDE:
Metformin is a commonly used first-line drug to treat Diabetes Mellitus type 2.
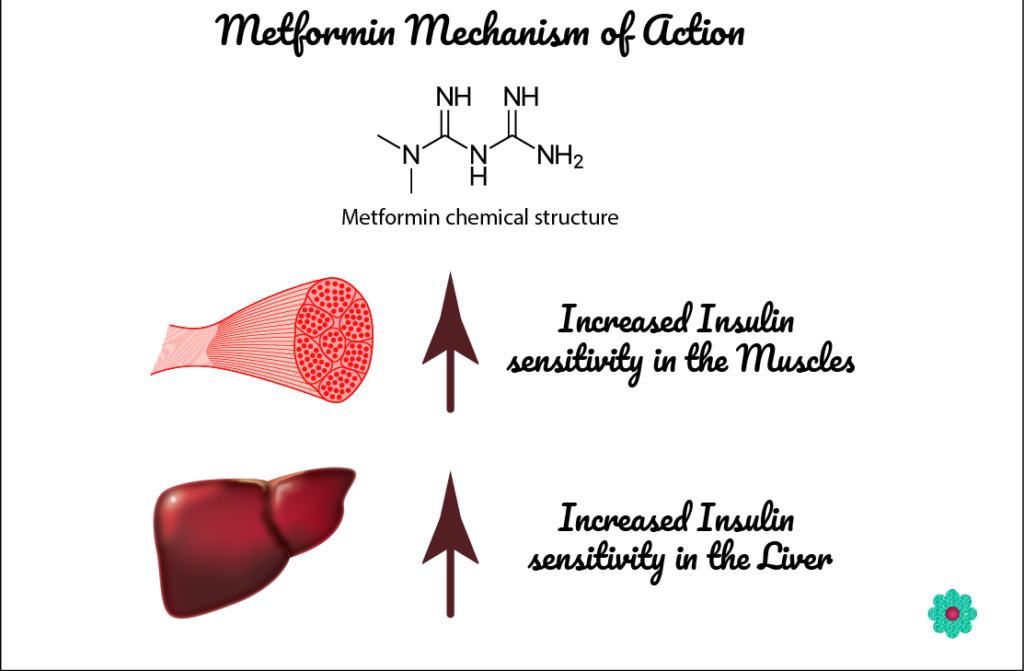
How does it work?
It works by elevating insulin sensitivity in the liver and body tissue cells and reducing glucose production by the liver.
It is metabolized by our liver and excreted from the body through urine. So it is not suitable for patients having kidney disease, and dose adjustments are necessary.
Uses:
- Metformin is used with diet and exercise to lower the risk of type 2 diabetes mellitus in obese patients when dietary and lifestyle modifications are not working for 3-4 months.
- Metformin is also used to treat high blood glucose during pregnancy.
- Metformin is used for type 2 diabetes patients when dietary changes are insufficient to control high blood glucose levels.
- Metformin is used in the treatment of obesity.
- It is also reported to reduce diabetes-associated complications.
Dosage:
- The starting dose of metformin is 500mg with the meal.
- It can be given 500mg twice a day with a heavy meal.
- The dose can be increased up to 2000mg per day.
- Metformin is also used safely in combination with other antidiabetics.
Side Effects of Metformin:
Metformin can cause side effects like all medicines, although not everybody gets them. The following side effects may occur;
- Diarrhoea
- Nausea
- Vomiting
- Bellyache or loss of appetite
- Metallic taste due to its secretion in saliva.
- Decreased vitamin B12 levels can be improved by supplementation with vitamin B12.
- Skin rashes, including redness, itching and hives.
- A rare side effect, “lactic acidosis,” may occur, a severe and life-threatening side effect that requires immediate hospitalization.
- Yellowing of skin and eyes: May cause abnormal liver function tests and hepatitis, which may lead to jaundice; contact your doctor immediately in this case.
Drugs that decrease insulin resistance – THIAZOLIDINEDIONES
In diabetic patients, either insulin is not producing, or there is not enough insulin, or the body develops insulin resistance to the already available insulin. More insulin is needed to reduce high blood glucose levels.
Pioglitazone & Rosiglitazone:
When taken, these reaches in blood within 30 minutes. And peak effect is seen within 2hrs of ingestion.
Taken with food delays its absorption but does not affect its blood availability.
Thiazolidinediones are metabolized in the liver and excreted in bile and faeces. So it can be safely administered to patients with kidney disease and the elderly.
It is contraindicated in patients with liver disease.
How do they work?
Thiazolidinediones produce their effect by decreasing insulin resistance. Low insulin secretion produced by pancreatic β cells results in a high glucose-lowering effect.
It also improves insulin sensitivity in our muscles and adipose tissues and inhibits the production of glucose by the liver. These depend on the presence of insulin in the body to be effective.
These bind with peroxisome proliferator-activated receptor-gamma (PPARg), which is found in the fatty tissues’ skeletal muscles and are responsible for insulin action. PPARg, upon activation, produces a series of changes in the number of genes responsible for glucose and lipids control in our body. These changes made by thiazolidinediones result in increased responsiveness of insulin-dependent tissues.
Thiazolidinediones increase insulin responsiveness decreases insulin resistance, hyperinsulinemia, hypertriglyceridemia in diabetes type 2 patients.
Thiazolidinediones consists of following agents:
- Pioglitazone
- Rosiglitazone
Both compounds have a similar mechanism of action, but they differ in their dosage, onset time and duration of action.
Dosage:
- The starting dose of Pioglitazone is 15mg per day
- The maximum dose of Pioglitazone is 45mg per day
- Starting daily dosage of Rosiglitazone is from 2 to 4mg per day
- The maximum daily dosage of Rosiglitazone is 8mg per day
- Both can be used as a single drug to treat diabetes mellitus type2 or in combination with other antidiabetics like insulin, metformin or sulfonylureas.
- Thiazolidinediones can be taken both with and without food.
Pioglitazone addition to the glucose-lowering effect, also decreased triglyceride levels and increased HDL cholesterol levels but did not affect total cholesterol in the clinical trials.
Rosiglitazone increases total cholesterol, increases LDL, increases HDL but have no effect on Triglycerides.
It also helps in recovering from non-alcoholic fatty liver disease.
Adverse Effects:
Both Pioglitazone and Rosiglitazone exhibits the same adverse effects. Which are
- Heart failure in patients receiving insulin therapy. (included in class III and Class IV cardiac status by New York Heart Association.
- Macular oedema.
- Increase fracture risk in women.
- Weight gain.
- Fluid retention.
- Pioglitazone increases the risk of bladder cancer.
Drugs that inhibit Glucose Absorption:
These drugs block the α-Glucosidase enzyme in the small intestine, which is responsible for the digestion of starch, maltase, isomaltase, sucrase, and glucoamylase, delaying the breakdown of sucrose and complex carbohydrates.
These drugs are known as α-Glucosidase inhibitors and include
- Acarbose
- Miglitol
Acarbose & Miglitol:
Both Acarbose and Miglitol inhibits the α-Glucosidase enzyme and stop the absorption of starch and glucose from the small intestine. The absorption of starch and glucose is delayed in the small intestine, and these pass to the large intestine.
This indigestion of starch and glucose causes the formation of gases by bacteria in the large intestine, which is considered one of the side effects of these drugs.
Dosage:
The starting dose for Acarbose is 50mg twice a day and can be increased up to 100mg thrice a day.
The starting dose for Miglitol is 25mg thrice a day, the maintenance dose is 50mg thrice a day and can be increased up to 100mg thrice a day.
Both of these antidiabetic drugs should be given with food.
Acarbose is not absorbed from the intestine and excreted through faeces.
Miglitol has structural similarity with glucose and absorbed from the intestine and is metabolized by the liver and excreted into the urine by kidneys, so contraindicated in patients having kidney disease.
Both of these causes a 30-50% decrease in blood glucose levels after a meal.
Side Effects:
The main side effects are the indigestion of glucose and starch from the small intestine, which causes the formation of gases by bacteria in the large intestine.
- Flatulence
- Diarrhoea
- Abdominal pain
- Hypoglycaemia occurs if given as a combination therapy with sulfonylureas biguanides. Hypoglycemia should be treated with dextrose as dextrose is not affected by these drugs.
These are not frequently prescribed due to their troublesome gastrointestinal side effects.
Drugs affecting Incretin effect :
The Incretin effect:
Usually, when we are ingesting anything sweet or made from sugar, a signalling mechanism is triggered inside our small intestine, which detects the presence of sugar.
The glucagon-like peptide 1 (GLP-1) hormone is released from the small intestine. GLP-1 goes into the bloodstream and shows its effects through its receptors on pancreatic β cells and cause the release of insulin, which controls our blood glucose levels. This effect is called as “Incretin effect” and is more pronounced when glucose is taken orally than intravenously.
In diabetic patients, GLP-1 secretion is reduced. Upon ingestion of sugar or sugar-containing products, GLP-1 is not released; in turn, adequate insulin is not released, and blood glucose levels remain high.
Apart from insulin secretion from the pancreas, GLP-1 also causes:
- Delayed gastric emptying
- Decrease feeling of hunger (satiety)
- Stops the production of glucose from the liver by inhibiting glucagon.
GLP-1 Receptor Agonists:
The life span of GLP-1 is short and is immediately metabolized by the DPP-4 enzyme and excreted out by kidneys. So to overcome this metabolism, GLP-1 agonists are synthesized, which have a longer duration of action, are not metabolized by dipeptidyl peptidase -4 (DPP-4), not excreted by kidneys immediately.
These acts on GLP-1 receptors on the pancreas and stimulate insulin release. It induces satiety, delays gastric emptying, and inhibits glucose production from the liver.
Following are GLP-1 receptor agonists, and are differentiated based on their duration of action, dosage, storage conditions;

Exenatide:
- Dosage: 5mcg immediately after breakfast and dinner.
- Directions: The dose can be increased up to 10mcg after one month of therapy, warning patients with impaired renal function.
- Duration of Action: 6 hrs.
Liraglutide
- Dosage:6mg or 1.2 mg, max 1.8mg 3mg per day only for weight loss.
- Instructions:6mg once a day can be increased to 1.2 mg per day after one week if there is no side effect and 0.6mg dose is not adequate. The maximum dose is 1.8mg.
- Duration of Action: 24hrs.
Albiglutide
- Dosage: 30mg to 50mg.
- Instructions: The starting dose is 30mg and can be increased up to 50mg after one week if the desired effect is not achieved.
- Duration of Action: 5 days. (discontinued from the market)
Dulaglutide
- Dosage:75mg to 1.5mg weekly.
- Instructions: The starting dose is 0.75 mg weekly by subcutaneous injection. The maximum dose is 1.5 mg weekly.
- Duration of Action: 1 week.
Lixisenatide
- Dosage: 10mcg to 20mcg daily.
- Instructions: The starting dose is 10 mcg daily by subcutaneous injection. The maximum dose is 20 mcg daily.
- Duration of Action: 24 hrs.
Semaglutide
- Dosage:25mg, 0.5mg to 1mg.
- Instructions:25 mg once a week for one month, then increase to 0.5 mg once a week if no side effects occur. The dose can be raised to 1 mg once a week.
- Duration of Action: 1 week.
Adverse effects of GLP-1 Receptor Agonists:
The most frequent side effects are:
- Nausea
- Vomiting
- Injection Site erythema (Albiglutide)
- Diarrhoea
- All the GLP-1 agonists increase the risk of Pancreatitis. If you have any abdominal pain, you should visit your doctor immediately.
- Exenatide also causes renal injury in some patients. If a patient has nausea, vomiting or diarrhoea, they must consult with the doctor.
DPP4 Inhibitors:
These inhibit endogenously released GLP-1 metabolism by inhibiting dipeptidyl peptidase -4 (DPP-4), resulting in a longer duration of action of GLP-1 and more insulin release from the pancreas.
Following are the available DPP4- inhibitors:
Linagliptin
- Dose: 5mg daily
- Duration of Action: 24 hours
Saxagliptin
- Dose: 5mg starting dose, maximum is 5mg
- Duration of Action: 24 hours
Alogliptin
- Dose: 6.25mg is starting dose, can be increased to 12.5mg if the targeted level is not reached, and the maximum dose is 25 mg
- Duration of Action: 24 hours
Sitagliptin
- Dose: 25mg is starting dose, can be increased to 50mg if the targeted level is not reached, and the maximum dose is 100 mg
- Duration of Action: 24 hours
Vildagliptin
- Dose: 50mg daily once or twice a day
- Duration of Action: 24hours
Adverse effects of DPP-4 inhibitors:
- Headache
- Allergic reaction
- Upper respiratory tract infections
- Urinary tract infection
- The FDA has issued a warning that DPP-4 inhibitors can induce joint problems that disappear if the medicine is stopped.
Benefits of DPP4- inhibitors:
These do not cause;
- Hypoglycaemia
- Alter gastric emptying
- Nausea
- Weight loss
- Affect satiety
Drugs that inhibit glucose reabsorption from the Kidneys:
Naturally, glucose is filtered by the glomeruli of the kidneys. Glomeruli is a section of kidneys where filtration of blood occurs. When glucose is filtered, it is then again reabsorbed by proximal tubules of the nephrons by the action of sodium-glucose Co-transporters (SGLT-2) and is added into the bloodstream again.
The drugs which inhibit this transporter from reabsorbing the glucose and entering back into the bloodstream are called SGLT-2 inhibitors. This inhibition of glucose reabsorption causes glucose excretion from the urine and reduces overall blood glucose levels. The excretion of glucose into the urine is called glycosuria. This includes;
- Canagliflozin
- Empagliflozin
- Dapagliflozin
Canagliflozin:
- Dose: 100 – 300 mg daily
- Instructions: Starting dose is 100mg once a day but can be increased to 300mg. Renal function is strictly monitored; in decreased renal function, the dose is reduced.
- Duration of Action: 24 hours
Empagliflozin:
- Dose: 5 – 10 mg daily
- Instructions: 10mg once a day is given; for patients with liver disease dose is reduced to 5mg per day.
- Duration of Action: 24 hours
Dapagliflozin:
- Dose: 10 – 25 mg daily
- Instructions: Starting dose is 10mg once a day but can be increased to 25mg per day if the targeted blood glucose level is not achieved.
- Duration of Action: 24 hours
Side effects
- Increase the cardiovascular risk
- increase risk of amputations of toes
- Not recommended in kidney patients as it increases creatinine clearance and eGFR rate
- Urinary tract infections
- Pyelonephritis
- Septicemia
- Volume depletion and risk of hypotension
- This decreases the bone mineral density, especially in the lumbar spine and hip bone, causing an increased incidence of fractures.
Miscellaneous Antidiabetics:
Other agents also cause and decrease in blood glucose levels. Still, they are not commonly used due to more powerful and more effective antidiabetic medicines, as discussed above. Their increased side effects severe side effects outweigh their benefit as antidiabetic.
Pramlintide:
It is available in injectable form and used in both Diabetes Mellitus type 1 and type 2.
- Dosage: for Diabetes Mellitus type 2, 60mcg dose three times a day with a maximum dose of 120mcg. Administered before meal
For Diabetes Mellitus type 1, 15mcg three times a day with a maximum dose of 60 mcg per day. If hypoglycaemia occurs, insulin dosage is reduced.
- Duration of Action: 2 hours.
- Side Effects: Decrease gastric emptying, Severe nausea, Hypoglycemia, Loss of appetite.
Bromocriptine:
- Dosage: 8mg daily. Maximum by 4.8mg per day. Increase one tablet per week.
- Duration of Action: 24 hours
- Side Effects: Nausea, Vomiting, Dizziness, Headache.
Colesevelam:
- Dosage: 625mg per day
- Duration of Action: 24 hours
- Side Effects: Not effective as monotherapy, Increased triglyceride levels, Constipation, Dyspepsia.
Ending Note:
Choosing an antidiabetic is not a simple task; we cannot simply select anti diabetics based upon their benefits. The choice of antidiabetic depends on pancreatic beta-cell insufficiency to produce insulin, insulin sensitivity and insulin resistance which is altered in diabetic patients.
A comprehensive diabetes treatment plan will include achieving optimal glucose control and meeting acceptable glycaemic targets and screening, prevention, and management of microvascular and macrovascular problems.
When controlling type 1 or type 2 diabetes, glycaemic control is crucial. It needs regular evaluation and modifications to diet, exercise, and pharmaceutical treatments.
In patients fulfilling treatment goals and on a stable therapy regimen, HbA1c should be tested twice a year. Individualized treatment is required based on diabetes and each patient’s particular needs.










